Safety of Mass Measurement in Pharmaceutical Industry. Basic Metrological Tests (part II)
.jpg)
Metrological tests increase mass measurement safety, provided that they are performed correctly and regularly. In the pharmaceutical industry, the measurement safety is a priority. With regard to this, the second part of our article will focus on the methodology of the metrological tests. First, we will tell you how to get ready for such tests.
Preparation Prior to Tests
Prior to tests it is required to get familiar with notions concerning the weighing instrument, mass standards and methodology. This shall allow to design such test cycle, which will clearly indicate measurement accuracy and precision.
Balance
Each balance features default settings on which it depends how fast and precise the measurement can be performed. The default settings guarantee correct balance operation in typical laboratory conditions, i.e., temperature of about 20oC, relative humidity of about 40%. Factory settings optimizing balance operation are adjusted on the basis of observation of mass standard weighing. In the course of laboratory weighing, objects other than mass standards are measured, these are bulbs, beakers, vessels etc. This is the reason why sometimes it is necessary to slightly modify the balance settings, when doing so, the real process specification must be referred to. In the case of Radwag instruments, such optimisation may be realised during routine tests performed within the validation process. There are actually two main optimization types:
- optimization for speed,
- optimization for measurement precision.
.png)
Figure 9. XA 21.4Y.A PLUS – stent application
Method of measuring signal filtering
- very fast / fast,
- average,
- sslow / very slow.
Stability criterion
- fast,
- fast and reliable,
- reliable.
Optimisation in favour of speed may give a little bit worse accuracy and precision. This is due to the fact that in this very case a stable weighing result is defined by:
- short observation time, and
- considerable weighing result variation,
therefore wrong value may be taken for a stable result.
In order to optimize the weighing process in favour of excellent precision, the following are usually required:
- long-lasting observation of the measuring signal,
- a very insignificant weighing result variation.
It must be stated that for weighing instruments with the reading unit d = 1mg, i.e., PS 1000.X2 (Figure 10), there are practically no big differences in terms of the measurement time or weighing precision prior to or after optimization. Enormous differences are noticeable in the case of balances with the reading unit smaller than 0.1 mg, e.g., MYA 4Y series microbalances.
.png)
Figure 10. PS 1000.X2 – mass measurement with readability of 1 mg
Product code: WL-218-0026
In search for an ideal solution regarding weighing speed and precision it is necessary to take into account the real requirements for the process realised in the laboratory. This not only shall allow to select a proper balance, but also to save test material, which can be costly. Theoretical dependence between the mass measurement time and precision for a typical laboratory balance of high resolution is presented in Figure 11.
.png)
Figure 11. Balance parameter optimisation
For most balances, especially those with a reading unit ranging between 0.01 mg ÷ 0.0001 mg, the shortest measurement time will be a cause of worse measurement precision. With regard to this, it is practically avoided to set short measurement time. Optimal mass measurement time in the case of most laboratory balances is about 2 ÷ 15 seconds, depending on the reading unit value. As a result of lack of standard-specified definition of "measurement time" notion we may come across various terms rather aiming to emphasize the marketing message than provide objective information.
Mass Standards
Dispensing a particular amount of a substance requires prior verification whether the balance indications are precise or not. For this purpose, balance adjustment is carried out (read section 4). Alternatively, it is possible to compare the displayed balance indications when the load resting on the weighing pan is a mass standard of known weight value, with the said known value. Either case provides information telling how precisely the substance mass is measured.
pIn the course of a periodical balance control, it is rarely analysed what is used for the tests, whether the balance is verified with a weight or a mass standard. There are a few significant differences between these two:
- nominal mass of weights is specified by regulations, whereas weight value of mass standards may be arbitrary,
- shape of weights is specified by regulations (OIML R111-1), whereas shape of mass standards may be arbitrary, it is selected to suit the anticipated use, like in the caseof an electronic weighing instrument where the internal adjustment weight matches the mechanical design of the balance,
- any object made of material which guarantees mass stability, features identification marks and calibration certificate with mass value and calibration uncertainty specified, and with information about preserved traceability, may be a mass standard.
The above leads to a conclusion that each weight may be a mass standard (calibration necessity), however not each mass standard can be a weight, e.g., disallowed geometrical dimensions.
.png)
Figure 12. Weight and mass standard – calibration process
Today, in Radwag Measuring Laboratory the procedure of mass standard calibration is performed automatically with use of automatic mass comparators and dedicated RMC software. Both components are proprietary Radwag solutions, allowing for a very high accuracy and precision of calibration processes.
.png)
Figure 13. Mass standard set
Product code: OK-501-0026
Test Methodology
Each weighing instrument can be tested by means of numerous methods, however it is not recommended to do so (too many information to be processed, time-consuming process, costly operations). The number of tests should be reduced to the very minimum, with this, only necessary information regarding balance status is obtained (valid / invalid). Nevertheless, the measurement result without any comment is useless, therefore while planning tests it is necessary to define:
- own expectations when it comes to conformity with critical limits (standard-specified, industry reference for the parameter under test, e.g., analysis accuracy by USP 41),
- test method, adequate for the balance scope of operations,
- what the test result means for processes performed in the laboratory,
- potential factors which may influence test result.
It is neither recommended to establish complex control procedures nor to carry out complicated tests with high intensity. Some control procedures may be realised automatically with use of internal balance functions such as, for example, report on balance adjustment, Autotest GLP. The first will inform about the weighing accuracy, the other one about the weighing precision. In both cases the internal adjustment weight is used. More accurate description of these procedures is to be found in later sections of this publication.
.png)
Figure 14. XA 82/220.4Y PLUS – powder weighing. GLP Report
Product code: WL-107-1029
From the perspective of Quality Management Systems, control procedures must be a tool for improvement, i.e., risk analysis process which shall be carried out in each organisation (PDCA).
Measurement Accuracy and Precision
Indication accuracy is a notion combining all the factors which influence the weighing result. Among them there is linearity, repeatability, eccentricity and variation of sensitivity. These factors altogether may cause imprecise balance indication.
Measurement accuracy is closeness of agreement between a measured quantity value and a true quantity value of a measurand (source: ISO/IEC Guide 99 International Vocabulary of Metrology. Basic and General Concepts and Associated Terms, VIM). Measurement accuracy notion is not a quantity (it is not given in a numerical quantity value). The measurement is more accurate when the measurement-correlated error is smaller (Figure 15).
.png)
Figure 15. Measurement accuracy
Error of measurement no. 2 (value 11) is greater than the error of measurement no. 1 (value 5), measurement 1 is therefore more accurate. Evaluation of mass measurement accuracy requires use of mass standard of known weight value. Example:
- mass standard weight 50.000165 g (calibration certificate)
- balance indication 50.0004
- error of balance indication accuracy 50.000165 – 50.0004 = - 0.000235 g = - 0.0002 g
- weighing of a sample of mass close to 50 g is carried out with an error of about - 0.2 mg.
Measurement precision is closeness of agreement between indications or measured quantity values obtained by replicate measurements on the same or similar objects under specified conditions. Measurement precision is usually expressed numerically by measures of imprecision, such as standard deviation, variance, or coefficient of variation under the specified conditions of measurement. The lower precision, the greater the standard deviation value.
Indication Repeatability – Measurement Precision
|
SOP |
REPEATABILITY |
|
Definition |
OIML R76 USP 41, USP 1251, European Pharmacopeia point 1.7.2 |
|
Equipment |
0.2 g, 10 g, 50 g, 100 g, 200 g mass standards |
|
Method |
Manual Load the weighing pan 10 times with a mass standard of a respective nominal value and record the weighing results. Balance indications for an unloaded weighing pan may be zero prior to and between the measurements. Balance adjustment is not required prior to the test start. Automatic – Autotest GLP Enter ,Misc.” submenu and run Autotest GLP function. The adjustment mass will be weighed 10 times. After procedure end, the balance will display the standard deviation value calculated for the measurement series. |
|
Limits |
Legal metrology, OIML – R 76: the permissible difference between the maximum and minimum indication cannot be greater than 5d ÷ 15d, this is conditioned by test load (read ANNEX 1). |
|
Interpretation |
The measurement is never accurate; however, it can be estimated with a certain probability where the measured value falls (3-sigma rule). Referring to the average value of the measurement series and to the standard deviation (S) it can be concluded that:
|
In stable conditions the precision is a permanent balance feature, therefore determining the above dependencies allows to specify whether the mass measurement exceeds the set limits for applied probability or not.
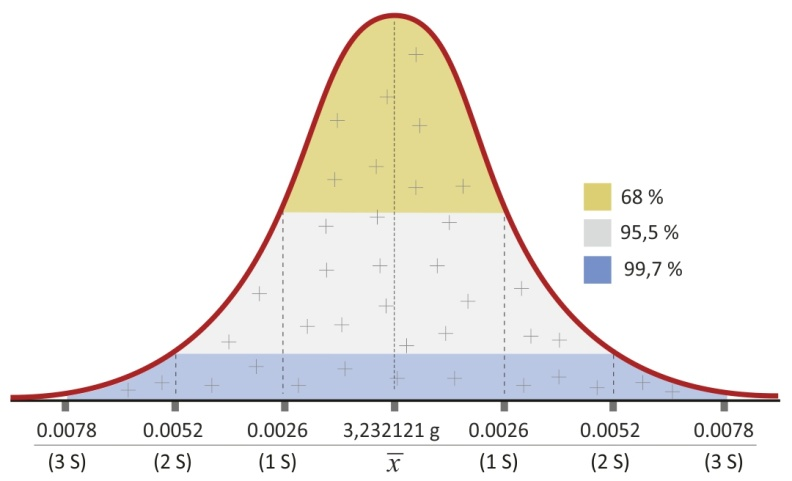
Figure 16. Standard deviation interpretation – 3-sigma. rule
Precision of Balance and Microbalance Measurement
The result of measurement precision test depends on three factors, namely thermal stability of the balance and the environment, operator skills regarding the art of weighing, and the applied test method.
Realizing the importance of these factors is the first step on a way to objective tests. Below, precision tests for two balances with different reading units are presented. The results have been referred to legal requirements (OIML R 76) and limits set by Radwag for the Quality Control.

Figure 17. AS 82/220.R2 PLUS
Product code: WL-104-1051
Remark
Measurement precision for AS 82/220.R2 PLUS balances complies with the requirements of the OIML R 76 and with the requirements of the Quality Management System of the Quality Control Department in Radwag.
The lowest value of the verification unit (e) by OIML R 76 is 1 mg. The value of the microbalance reading unit (d) is 1 mg. Within the low limit area of the weighing range, the maximum permissible error of accuracy (MPE) takes 0.5 of the verification unit value, that is 0.5 mg. With regard to the above, the mass measurement error may be up to 0.000500 g. For this very reason testing the microbalance metrological parameters in accordance with legal regulations (OIML) is not recommended.

Figure 18. MYA 5.4Y PLUS Microbalance
Product code: WL-101-0203
Eccentricity
|
SOP |
ECCENTRICITY |
|
Definition |
OIML R76 |
|
Equipment |
Mass standards of nominals close to either ⅓ or ½ Max capacity value of the tested instrument |
|
Method |
Manual (OIML R 76)
Manual – differential
|
|
Limits |
Legal metrology, OIML – R 76: the permissible difference between balance indication for a control spot shall not be greater than the value of an error for a particular load (ANNEX 1). Area not regulated by law: the maximum difference for control spots 2 ÷ 5, calculated with regard to indications in the centre point of the weighing pan shall not be greater than the value given by the manufacturer (product datasheet). |
|
Interpretation |
The Good Laboratory Practice guidelines recommend to place the weighed objects in the very centre of the weighing pan. Therefore, the potential error of eccentricity is not of a significant importance. An exception may be objects with shift in the centre of gravity. |
In the case of most laboratory balances manufactured by Radwag, the error of eccentricity is about 3 reading units. For the evaluation, a mass standard of weight value of ½ Max capacity is used.
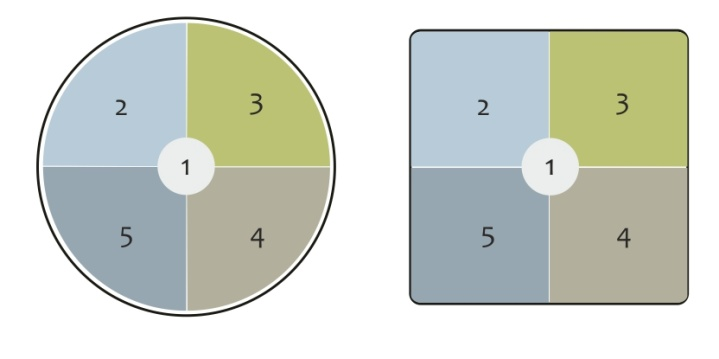
Figure 19. Spots for control of the eccentricity test
Differential Error of Eccentricity
Differential error of eccentricity is a deviation between the result obtained while weighing a mass standard located successively on spots 2 ÷ 5 and the result obtained while weighing the same mass standard when placed centrally on spot 1 (Figure 19). Formula:
Ecc = I(1) – I(i)
where: Ecc – differential error of eccentricity
I (i) – indication for non-central spot (2, 3, 4, 5)
I (1) – indication for the central spot
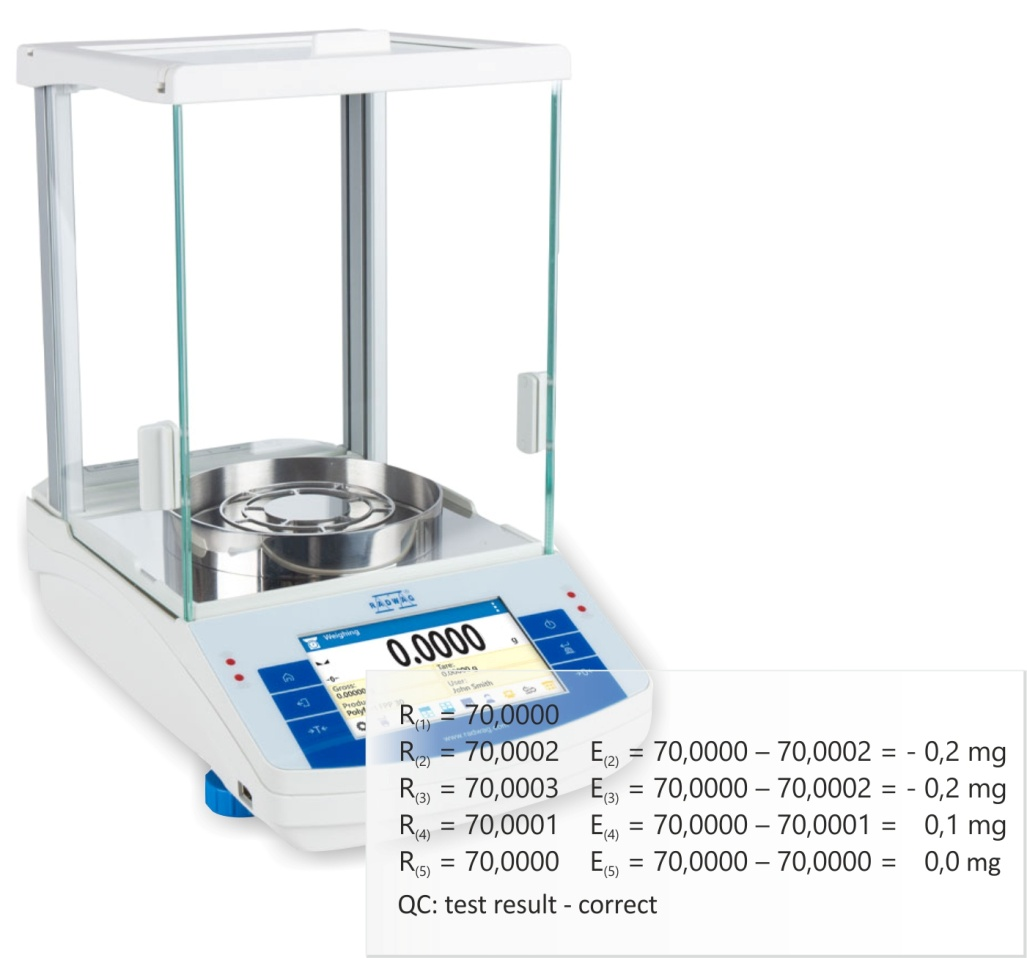
Figure 20. AS 220.X2 balance – eccentricity test
Product code: WL-104-0169
Eccentricity is a parameter of a constant value, therefore there is no need to test it too often. In practice, the eccentricity test is reasonable only when weighing large mass samples (over ½ Max). This parameter is not important for small masses, the influence of repeatability is predominant. Should the parameter be controlled then?
The eccentricity should definitely be checked after balance installation. The test result will let to judge whether the balance transport caused any changes in balance characteristics or not. In the course of operation this parameter value is constant, therefore the control should take place periodically (with a long interval, for example, every few months).
Linearity
The linearity parameter determines the difference between a weighing result and a reference value, i.e., the weight of the mass standard. When it comes to the linearity, the whole weighing range is evaluated, however sometimes it can be reduced to just a part of it. A perfect weighing instrument is such an instrument that enables 'precise weighing', which means a weighing guaranteeing that the indication and the weight value provided on the calibration certificate are compliant. The precise weighing is presented by the green line, the dashed line stands for non-linearity (Figure 21).
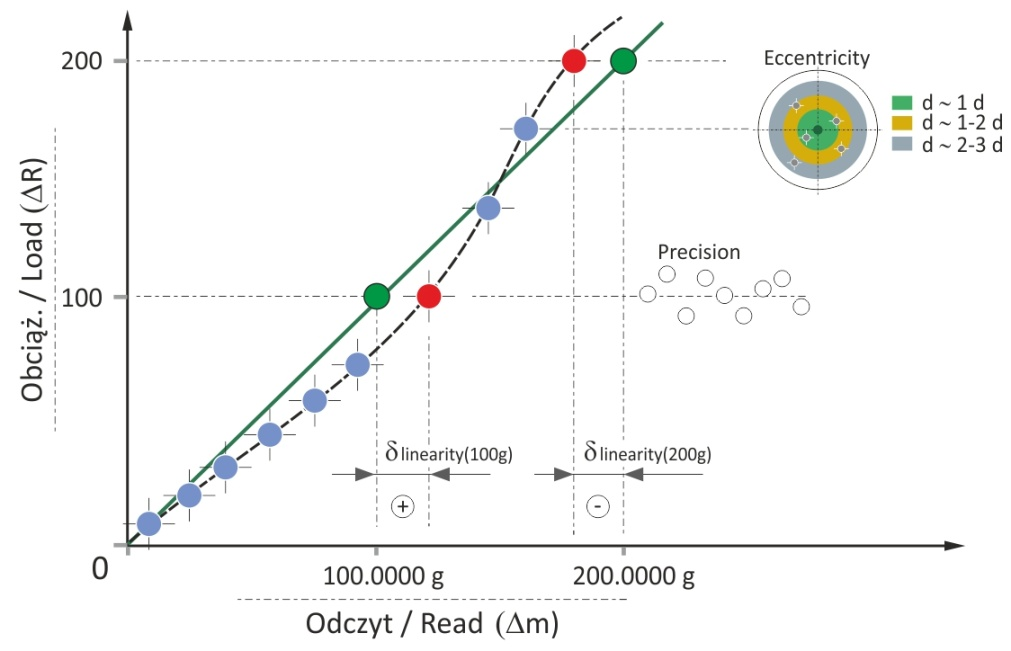
Figure 21. Balance linearity – the model balance
Balance non-linearity may be a result of errors of mass standards used in the course of factory adjustment, imperfect measurement methods, metrological possibilities of the balance and operator mistakes. In fact, the linearity deviation accumulates other errors, for example ones resulting from the measurement precision or the eccentricity. It may be said that the additional components contributing to the linearity deviation budget depend on the weighing range. For sample mass comprised within the range from 0 to ½ Max capacity, the linearity deviation may be significantly influenced by:
- measurement precision, i.e., repeatability (influence of ambient conditions, skills etc.),
- mass standard error, too high uncertainty of determination of the mass standard weight, mass standard dirtiness, etc.
For sample mass comprised within ½Max ÷ Max range, the measured linearity deviation may be significantly influenced by:
- measurement precision, i.e., repeatability (influence of ambient conditions, skills etc.),
- eccentricity error,
- mass standard error, too high uncertainty of determination of the mass standard weight, mass standard dirtiness, etc.
An attempt to reduce these errors is a never-ending story, it is done with the help of a respective methodology, etc., special holders intended for the weighing vessels (read section 3), ambient condition monitoring, personnel training, other. While selecting a balance for a particular application, the potential errors should be considered, with this, safety of the processes in the laboratory will be preserved.
Analysis of balance linearity deviation must also account for the fact that weighing of real objects such as powders, bulbs, vessels, extraction thimbles may be burdened with a greater error. This error may be caused by sample instability (absorption/desorption), occurrence of too many static charges, thermal instability of the sample. Weighing methodology must take into account such processes and indicate means of risk elimination.
In practice the linearity assessment involves adjustment performance (Figure 5). Usually, the internal adjustment mechanism is used for this purpose. Such process eliminates balance sensitivity error, which may be a result of an ongoing thermal stabilisation of the balance, transfer of the balance from the production to operation, other environmental conditions. The adjustment may as well be performed using the external mass standards, however in such a case it is necessary to remember that the real mass standard weigh is its nominal mass after consideration given to the deviation (see the calibration certificate). Figure 22 shows an example of balance adjustment report.

Figure 22. MYA 21.4Y PLUS – powder weighing, adjustment report
Product code: WL-101-0414
|
SOP |
Linearity |
|
Definition |
OIML R76, ISO 5725-1 (accuracy) |
|
Equipment |
|
|
Method |
Manual (OIML R 76)
Manual – with use of supplementary weights
|
|
Limits |
Legal metrology, OIML – R 76: the permissible difference between balance indication for a control spot shall not be greater than the value of an error for a particular load (ANNEX 1). The supplementary weight method – the permissible difference between balance indication for a control spot shall be neither greater than the value of an error for a particular load (ANNEX 1) nor the value given by the manufacturer (product datasheet). |
|
Interpretation |
Use of mass standards within the whole weighing range may be problematic due to the uncertainty of mass standard weight determination. Use of the supplementary weight method is based on the assumption that regardless of the used supplementary load, mass measurement of the same mass standard shall provide the same weighing result, the load / indication dependence is ideally linear (Figure 21, green line). |
Linearity – Legal Metrology
In accordance with the requirements of OIML R 111-1, OIML R 76, error of the weight used during the metrological tests cannot be greater than ⅓ of maximum permissible errors for the given load (ANNEX 1). For this reason, testing balances with very small reading units, like for example the XA 4Y or MYA 4Y series, where d < 0.01 mg, may not provide an objective information on balance accuracy / linearity. For device of accuracy class II and III such problem does not exist since for the test purpose, weights of accuracy class F2 are used.

Figure 23. PS 1000.X2 with mass standard set – test of balance indication accuracy
The balance complies with the requirements of the legal metrology and the requirements of Quality Management System adopted in Radwag.
Linearity – The Supplementary Weight Method
This method requires use of one mass standard and a respective quantity of supplementary weights.
.png)
Figure 24. Metrological control of AS 220.R2 PLUS series balance
Product code: WL-104-0177
While designing the control tests it is necessary to account for a respective test quantity and sophistication level. Only those areas and functionalities that are significant for the quality of laboratory operations shall be checked, to the necessary extend. It is also necessary to bear in mind the fact that each object of mass constant over time can serve as the mass standard.

.jpg)











