Safety, Compliance with Regulations, and Economy. Which Weighing Instrument to Operate in Pharmaceutical Industry?
Balances used in pharmaceutical industry must meet few basic requirements, namely they should enable cost reduction, measurement safety, data integrity and compliance with regulations, they should also communicate with external software. Which models of balances manufactured by RADWAG offer these features and are used at particular production stages in pharmacy?
Balance Types Used in Pharmaceutical Industry
Cost reduction, measurement safety, data integrity and compliance with regulations, plus communication with external software - these are criteria to be fulfilled in pharmacy. What exactly does the above mean?
1. Cost Reduction
RADWAG balances are famous for the smallest minimum weight worldwide. This means that a very small sample is measured, which due to its size requires lower budget. In pharmacy such small samples are weighed in a number of few hundreds a day, just imagine the monthly, quarterly, yearly savings!
2. Measurement Safety and Data Integrity
RADWAG balances feature modules which enable users to program the device so that it reminds them about scheduled routine tests, checks and other important dates. With this, on the exact day, a respective on-screen prompt is displayed. Regularly carried out tests ensure weighing accuracy - indispensable especially in such branches of industry as pharmacy.
Additionally, RADWAG balances offer wide selection of easily accessible databases. Device settings are fully customized, thanks to this the balance suits particular user needs and measurement type. In Laboratory there are many users, each of them can be assigned with an individual profile, with a preferred language of the interface, quick access keys selection and memory-saved working mode settings.
Due to the weighing process customization, the user receives unlimited control over both the weighing process itself and the device.
3. Compliance with Regulations
RADWAG balances comply with 21 CFR part 11, GMP, GLP and USP regulations, which means that they meet all necessary requirements guaranteeing correct weighing in pharmacy. Notably, the 4Y PLUS device offers full compliance with the requirements of 21 CFR part 11 regulations, as the only one worldwide, without implementation of any additional software.
4. Cooperation with External Software
RADWAG balances are compatible with external software like LIMS or WERUM, dedicated for laboratory processes and pharmaceutical and biotechnological branches.
Balance Types Used in Pharmaceutical Industry at Particular Production Stage
In pharmacy, each production stage requires use of a different balance type. What type exactly is used and where?
1. In-Warehouse Product Receipt and Onto-Production Dispatch
First, the raw material is weighed. The system provides a detailed description of each raw material. For each raw material a label is generated. The label data informs, among many, about raw material expiry date. A particular quantity of given ingredients is dispatched onto the production line.
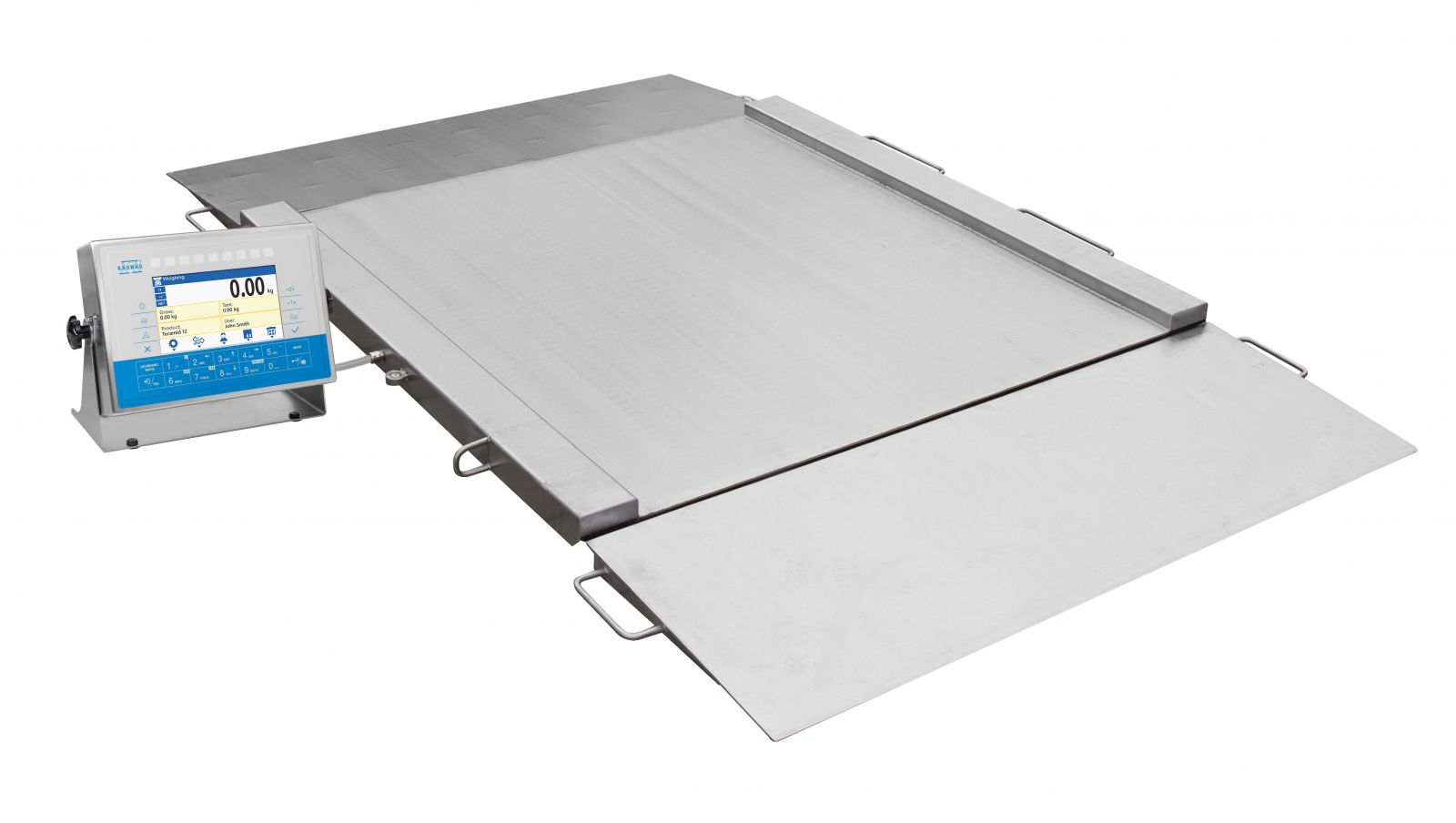
At the stage of in-warehouse product reception, and at the stage of product transfer to the production, only stainless steel scales, for example ramp scales (300 kg), are used.
These are industry 4-load platform scales, with use of which you will measure weight of heavy loads. 4 transducers guarantee precise mass measurement regardless of the position a load takes on the platform. Ramp scales are equipped with weighing pan wings of which can be opened, this makes the cleaning easy.
Other devices used at the production stage are 1-load-cell scales (15-30 kg) and instruments intended for hazardous areas, as well as peripheral devices such as labellers and scanners. Weighing equipment operated in pharmaceutical industry must be accurate, durable and easy-to-clean so that it could meet the highest hygiene and technology-related standards.
2. The Laboratory and the Quality Control
The Laboratory and the Quality Control department supervise the entire production process.
The test sample gets subjected to quarantine. It undergoes long and tedious tests and trial period. In the case of passed tests, the product is put onto the market.
Inter operational control consists in weighing of particular ingredients and formula making. Samples can be weighed in various ambient conditions and environments.
Formulation is a key working mode in the case of balances used in pharmacy, it supports the process of mixture preparation. This mode enables control of weighing, with a permissible tolerance, of each ingredient.
Formulation mode is available in most RADWAG balances, especially in those that are operated in the laboratory and at the quality control stage, namely in laboratory balances. Laboratories are often equipped with balances featuring an in-built ambient condition sensor, so called THB sensor.
3. Production and Packing
Each pharmaceutical company follows self-designed procedures at the stage of production and packing and therefore providing one practice is simply impossible. However there is one thing all the customized procedures have in common: complete control of each weighing process. Thanks to a detailed control the product leaving the production line meets the highest quality standards.
Some pharmaceutical companies have few separate control workstations and each of them is equipped with a balance or few balances of different accuracies with use of which the measurements are carried out. The next balance in line is used to determine the final product mass. Blister packs are checked with regard to mechanical contamination content, therefore they are passed through special contamination detectors.
 Packaged products ready for sales are subjected to check-weighing aiming to verify whether mass of a single pack of tablets from a cumulative package is of a specified value or not. Weighing of a cumulative package allows to check if there are any packs missing. Cumulative package is signed and provided with stickers, this makes it clear how many single packs are stored inside.
Packaged products ready for sales are subjected to check-weighing aiming to verify whether mass of a single pack of tablets from a cumulative package is of a specified value or not. Weighing of a cumulative package allows to check if there are any packs missing. Cumulative package is signed and provided with stickers, this makes it clear how many single packs are stored inside.
At the production and packing stage, high resolution platforms are broadly used. The HY10.HRP scales by RADWAG are unique due to the fact that although being industry devices they offer laboratory precision, this is by cause of use of 
Other useful weighing instruments at this stage are ramp scales of pit version, dynamic scales (installed along with metal detectors), multifunctional and PGC scales (PGC for weighing of cosmetics and supplements). Peripheral devices so much needed at these production stages are automatic feeders and labellers.
Balances used for production and packing purposes must comply with 21 CFR part 11, GMP, GLP and USP regulations.
Pharmaceutical branch employee? Want to buy one of the weighing instruments but have no idea which one to choose? Write to our expert, he will help to make the right choice.